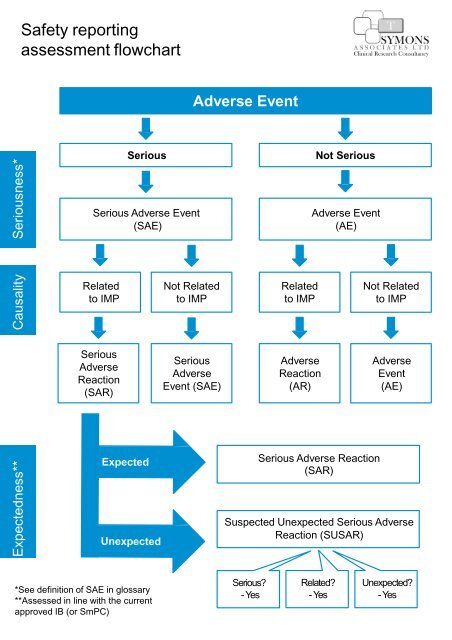
1 Nuts and Bolts of Safety Reporting The Role of the CRO Dr. Noa Lowenton Spier Pharma-Clinical S.A.G. - ppt download
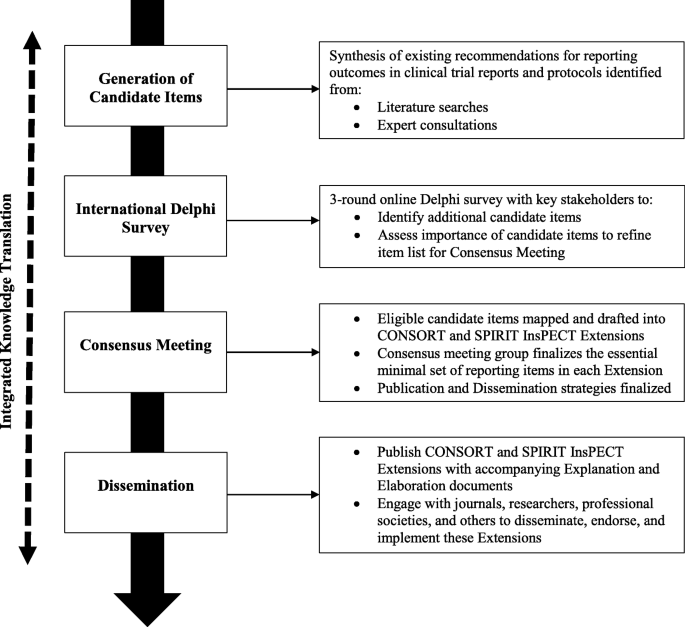
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text

Months and Severity Score (MOSES) in a Phase III trial (PARCER): A new comprehensive method for reporting adverse events in oncology clinical trials - eClinicalMedicine

Randomised Clinical Trials: Design, Practice and Reporting: 9781119524649: Medicine & Health Science Books @ Amazon.com

Safety Reporting Overload in Clinical Trials: FDA and Site Perspectives on Overreporting of Adverse Events | CenterWatch
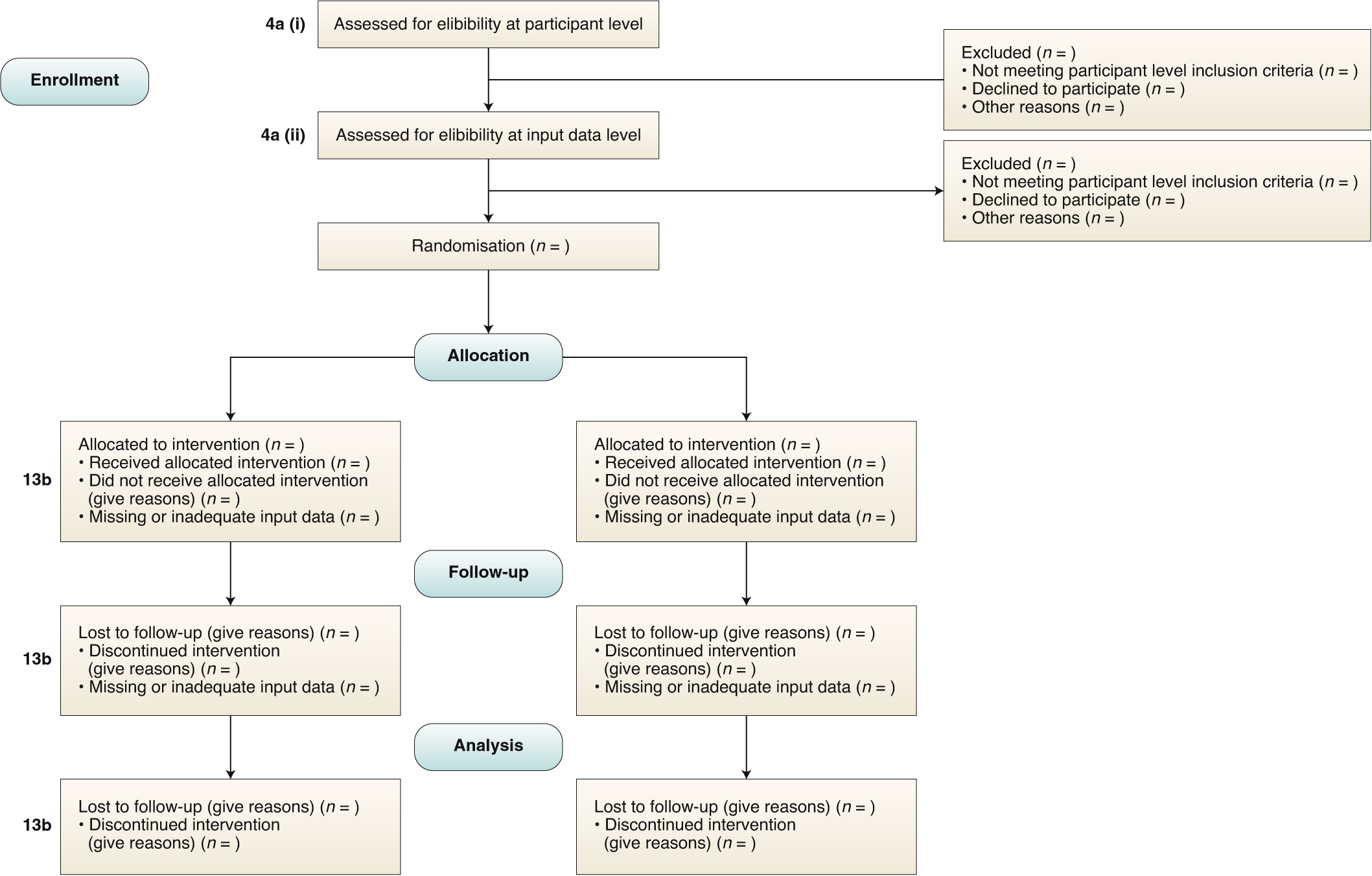
Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension | Nature Medicine